
Our Work in Life Science
Accelerating Success Across the Life Sciences Spectrum with Strategic and tactical assistance that leverages today’s technologies.
Astrix is proud to enter our third decade as a valued partner in powering our clients’ data-driven and technology-enabled successes.
Spanning the Life Science Spectrum
From exploration, discovery, and production to monitoring and regulatory compliance, we offer incomparable expertise to clients in:
- Biotechnology Manufacturing
- Biomedical Research
- Clinical Care
- Medical Devices
- Pharmaceuticals
Our experts bring exceptional insights and expertise that our clients find essential to achieving their objectives and optimizing their mission-critical operations including:
- Research & Translational
- Nonclinical Development
- Clinical Development & Clinical Trial Management
- Regulatory, Safety, and Manufacturing
- Strategic Planning, Roadmaps & Process Optimization
From enhancing digital readiness to assisting in the evaluation, selection, implementation, and customized optimization of new software suites and tools, Astrix people are dedicated to measurably improving our clients’ capabilities:
- Strategic Innovation
- Integrated Business Analysis
- Technology & Architecture
- Digital Management & Governance
- Scientific & Technical
How We Enable & Optimize Life Science Operations
Laboratory Informatics
The Astrix Approach™ is a vendor-agnostic, proven methodology designed to ensure success in laboratory informatics projects. Astrix has developed and refined this blueprint for success based upon more than 25 years of researching best practices and implementing, observing, and measuring results for scientific and laboratory-based organizations. We offer a comprehensive suite of services to help labs drive success through greater efficiency and visibility into the data that drives their projects.
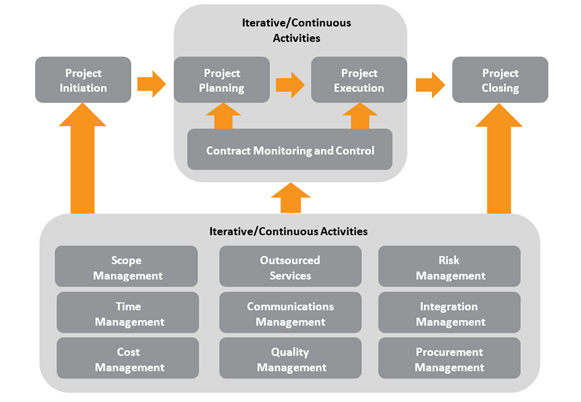
Program Management
A system implementation can be complex to navigate and align with your organization’s business goals while tailoring the functionality to your unique laboratory environment. Astrix maximizes the return on your investments while ensuring the system’s successful deployment by developing and executing a thoughtful program management strategy that leverages appropriate technology, resources, and processes. A system implementation can be complex to navigate and align with your organization’s business goals while tailoring the functionality to your unique laboratory environment. Getting this done right the first time is critical to the long-term success of your operations. Utilizing an experienced program manager from the outset of your project through execution can dramatically improve timeframes, deadline achievement, budget adherence, and outcomes of use.
Our Implementation Methodology
Business Process Analysis
At Astrix, we apply Business Process Analysis (BPA) as the first step in our comprehensive and proven blueprint for success. Astrix Business Analysts work to align business processes, business goals, and technology to ensure your laboratory informatics project maximizes business value for your organization. Our rigorous approach to BPA has three main objectives:
- Accurately capture the current state environment to create an “As-is” process map.
- Design the future state workflow with user buy-in, leveraging the latest technology and functionality to optimize process improvements, identify risks, and create a “To-be” process map.
- Develop precise requirements that reflect agreed-upon improvements and focus vendor evaluations on those requirements – Vendor Selection.
Informatics Roadmap Development
Leveraging the process maps and the Business Needs Assessment, Astrix will weigh these factors, discuss with the stakeholders, and leverage industry best practices to propose an informatics roadmap that most efficiently meets the needs identified in the Business Needs Assessment. Our ultimate goal is to provide the stakeholders with an informatics roadmap that meets their present needs and future demands to deliver maximum value for their organization.
The final Roadmap will include the following:
- A recommended 3 to 5-year informatics strategy.
- Candidate high-level architectures with recommendations.
- Structure for projects to reach the recommended architecture.
- Project approach recommendations and high-level timelines.
- High-level internal and external resource requirements.
Technology Selection
Using an extensive evaluation process, Astrix experts review all prospective vendors that fit the customer’s requirements and budget, examining every potential option. Our vendor-agnostic approach means that our selection is impartial and unbiased, allowing us to provide objective advice on which vendors should be considered. Not only are clients assured of Astrix independence thanks to our policy of abstaining from all forms of vendor compensation, but system recommendations are accompanied by a rationale for each selected vendor. This ensures that our reasoning for a specific solution is transparent in its explanation of its benefits over other options.
We are committed to delivering a list of vendors that is informative, thoroughly researched, and includes all viable options within the confines of the customer’s project requirements and budget.
The technology selection process is comprised of these elements:
- Request for proposal (RFP) development
- RFP response scoring and review
- Vendor demonstration script/agenda preparation
- Vendor demonstrations
- Vendor selection discussion
System Implementation and Deployment
We understand the imperative for systems implementations and deployments to go smoothly, so we take care in every step of our process – from requirements gathering through final deployment – to ensure success. Our expert teams are composed of engineers, developers, testers, and subject-area experts who leverage their extensive experience in implementing business solutions. They provide technical insight and knowledge about industry-specific regulations and standards that may need to be followed for successful delivery.
Astrix is committed to success with each system implementation and deployment in terms of alignment with our client’s processes and procedures. Successful implementations involve extensive planning, testing, and stakeholder communication. Our methodology is tailored to each engagement and typically involves four key steps: discovery & assessment; design & build; test & optimize; and launch & support as outlined in these program milestones:
- Project Initiation
- Requirements Analysis
- Gap Analysis
- System Configuration and Integration
- Master Data Management and Migration
- System Testing
- Training
- System Deployment
- Go-Live Support
Validation for Regulatory Compliance
We identify and manage IT risks to ensure that computer system validation (CSV) processes comply with the latest FDA regulations and guidance. Our CSV methodology is risk-based and tailored to the technological, operational, and business risks associated with each client’s systems.
This enables us to tailor the tests used in CSV accordingly and provide a level of effort that meets industry standards and best practices. By leveraging our experience and expertise, we can provide comprehensive validation services that are robust yet agile enough to accommodate changes in regulatory requirements or other factors and are designed to maintain compliance throughout the system’s lifespan.
Astrix’s CSV processes typically involve:
- System inventory and assessment
- User requirement specifications
- Validation plan
- Risk assessments
- Functional requirement specifications
- Network and Infrastructure Qualification
- IQ/OQ/PQ Scripts and Results
- Validation Report
- System Release Documentation
Some of our Clients Include





















LET´S GET STARTED
Contact us today and let’s begin working on a solution for your most complex strategy, technology and strategic talent services.
CONTACT US