CASE STUDY
Optimizing CMC Processes Empowers Cell Therapy Company Growth
Overview
The pace of development for cell and gene therapy (CGT) relies heavily on the efficiencies in chemistry, manufacturing, and controls (CMC). However, the unique characteristics associated with managing this new breed of therapies—especially their quality and tracking controls—pose unique challenges that encompass:
- Short shelf life relative to pharmaceutical products
- Diversity in starting and supplementary materials
- Manufacturing complexity
- Logistics
- Cost
Given the nature of these innovative therapies, it is not surprising that CMC-related questions are more frequently included in FDA CGT information requests (IRs) than non-CGT therapeutics. This case study sheds light on how one company specializing in cell therapy leveraged Astrix’ expertise to produce an effective roadmap for growth based on aligning priorities, and projects, through the development and documentation of cohesive CMC:
- Vision
- Business process
- Technology strategy
The engagement with Astrix resulted in the biotech’s leadership team clarifying its priorities and achieving alignment on its project-based approach to empowering its growth.
Business Challenge
A biotech company specializing in cell therapy was growing rapidly and experiencing a critical need to scale its IT infrastructure to support the business. The personalized immunotherapy sector is delivering a relatively new therapy platform. Manufacturing processes are evolving rapidly and are characterized by challenges that are both new and unique.
Since individually tailored therapy equates to one set of batch records for each patient, data requiring careful management can multiply rapidly. This is particularly crucial during clinical trials when batch associated with only 50 to 100 patients are run.
Then, to ensure the manufactured product is transfused to the right patient, it is imperative to ensure ironclad chain of custody (CoC) and chain of identity (CoI) tracking throughout the supply chain.
Since many of the client’s business processes were evolving, their technology solutions were difficult to plan. As a result, much of the CMC area was running on paper and spreadsheet stop-gap methods. The leadership team knew that paper-based process
would not keep pace because of time-consuming and error-prone manual processes. The company needed enterprise-wide technology solutions with investments in the highest priority areas on a “first- things-first” basis. That is, they needed a strategy that they could execute against in a pragmatic way gated by the progression of the business.
How Astrix Enabled Success
The company turned to Astrix to create the strategy to outline the high-level business processes and to capture required business capabilities. This would involve working with the senior leaders who knew their issues and gaps, but just didn’t have the time to design, organize and drive a program structured to scale the business. With limited documentation on which to rely, Astrix met with leadership to gain further insight into their challenges and needs across the CMC area. The focus was on:
- Demand forecasting
- Capacity and supply planning
- Order scheduling and management, inventory and warehouse management
- Manufacturing execution and critical production, quality parameters
- End-to-end patient/product supply chain management
- CoC/CoI sample tracking
- Overall integration of data for process development purposes.
Using senior leadership’s input, Astrix documented a strawman future state environment with foundational CMC processes and key business capabilities.
The figure below illustrates the resulting high-level business process (generalized from the client environment). Each of these high-level process steps were detailed in successive layers of swim lane diagrams.
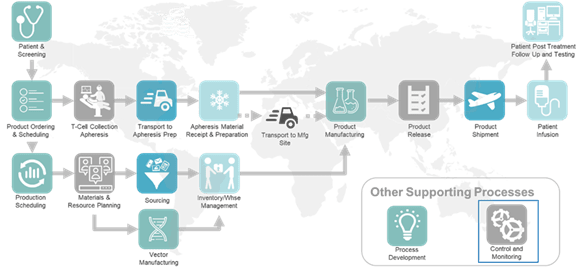
Based on the definition of the future state business flows and capabilities, the company also needed an information management strategy to support the envisioned scale and to better leverage data generated throughout the cell therapy lifecycle. They needed to balance the need for significant short-term business improvement and ensure the ability to support near term milestones while providing a foundation for long-term business growth and success. A high-level conceptual
information architecture and data flow was designed to facilitate this and to support the agreed upon business processes. This led to a prioritization of technical capabilities that were captured in an information management roadmap to guide investments over the ensuing two to three years.
Subsequent efforts, based on the roadmap, focused on year-one technology projects starting with detailed definition of requirements and conducting the corresponding solution selection activities.
