
Case Study: Structured Content Management Strategy in a regulated clinical trial environment
KEY BENEFITS
Organized and Optimized entire document management process
Developed 2-3 year look-ahead roadmap to ensure technology infrastructure keeps pace with corporate objectives
SOLUTION
Clinical
INDSUTRY
Pharmaceutical
Structured Content Management Strategy for a Global Pharmaceutical Company
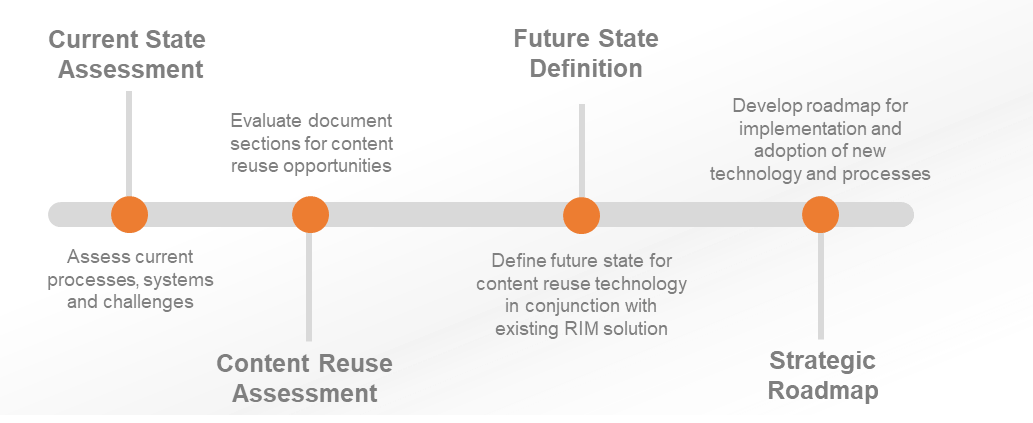
Challenges in regulatory document authoring and management are common for many global pharmaceutical companies, where document flow and content reuse must be optimized to ensure compliance and improve user experience. For one such company, an assessment of their current state revealed the need for increased alignment between their RIM and structured content management programs. With over 30 regulatory document types in need of optimization, key stakeholders sought to establish a future state process for leveraging content reuse technology within their existing RIM platform. Through a collaborative effort, an implementation roadmap was developed to achieve the targeted objectives. This case study outlines the process undertaken, highlighting the successes achieved and lessons learned along the way.
Business Objectives
- For a global pharmaceutical company – establish alignment across RIM and Structured Content Management programs to ensure complete document flow and improve the user experience from first draft to final approval
- Assess the current state of regulatory document authoring, including high-level processes and systems interactions
- Assess content reuse opportunities for 30+ regulatory document types
- Define a future state process for leveraging content reuse technology in conjunction with existing RIM Platform
- Define an implementation roadmap to achieve the future state of SCM & RIM
Results Delivered
- Aligned on a common understanding of current document authoring processes and challenges
- Detailed content reuse opportunities for 30+ document types across Regulatory, Safety, and Clinical
- Developed a matrix depicting upstream and downstream content use by document section
- Prioritized document types for next steps (piloting SCM technology)
- Designed a future state process enabling use of various structured content management tools and technology in conjunction with existing RIM platform
- Developed a 2-3 year strategic roadmap outlining activities supporting technology implementation, business process evolution, User Experience optimization and Organizational Change Management
LET´S GET STARTED
Contact us today and let’s begin working on a solution for your most complex strategy, technology and staffing challenges.
CONTACT US


