CASE STUDY
Case Study: Regulatory Information Management
RIM Migration and Management for a Global Pharmaceutical Company
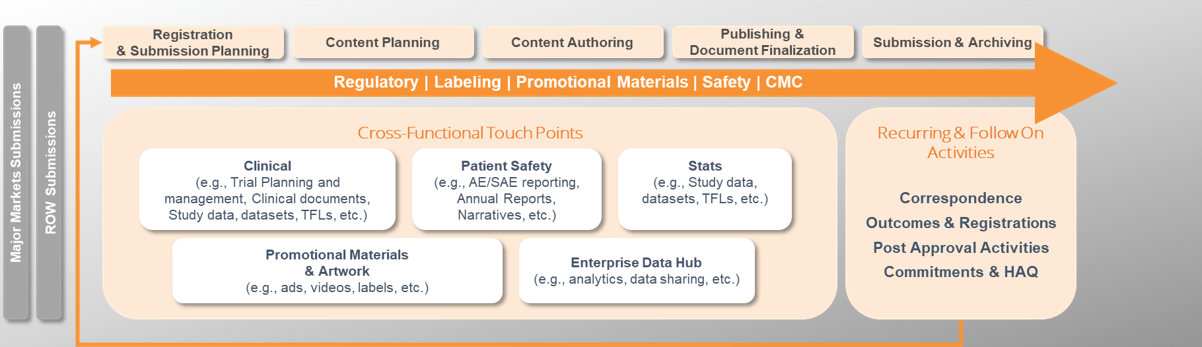
A major pharmaceutical company was faced with the challenge of streamlining its regulatory submissions process globally. They required a solution that would harmonize their planning, tracking, authoring, publishing, submission, archive, and correspondence with Health Authorities, and selected Veeva RIM as the technology platform to support this transformation. To ensure a seamless transition, they engaged Astrix, having extensive experience in regulatory information management systems (RIM), to plan and manage the migration of data and documents from multiple legacy systems, aligning with phased-release plans and the RIM roll-out strategy.
Business Objectives
- Define processes and support configuration a globally harmonized solution for regulatory submissions planning, tracking, authoring, publishing, submission, archive, and correspondence with Health Authorities.
- Plan and manage migration of data and documents from multiple legacy systems aligned with phased release plans and RIM roll out strategy
- Plan and manage integration workstreams to support transfer of data and documents to/from Veeva RIM
- Plan, manage, and implement Organizational Change Management activities to successfully onboard users to Veeva RIM
Results Delivered
- Moved outdated unconnected legacy Regulatory Affairs systems to a unified Regulatory Information Management platform
- Increased visibility and alignment across Regulatory Affairs corporate, affiliates and safety functional areas
- Cleansed data and content and migrated legacy Regulatory information from a myriad of systems, spreadsheets and file shares to the new platform
- Planned and executed adoption and organization change management activities across the program
Managed the overall program over 5 years to ensure a successful implementation and user adoption
