
Pharma 4.0 and Validation 4.0 – The Challenges of Implementing
Pharma 4.0™ and Validation 4.0, as part of the plan, requires resources, information technology, a holistic control strategy, and a cultural shift of the organization. All areas need to come together to ensure that the business can make progress towards improvement and achieve the objectives. This can lead to challenges given the extensive impact on the organization.
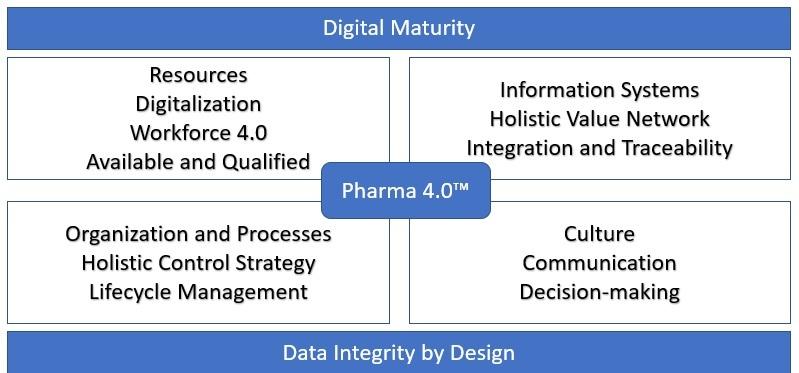
Challenges Impacting the Implementation of Pharma 4.0 and Validation 4.0
People, Culture, and Skillset Challenges
Manufacturers and regulators will need to make cultural adjustments and innovate to manage the myriad of data, computing, and automation hazards on the way to full adoption of Pharma 4.0™ and Validation 4.0 guidelines and the technologies required to get there. In order to achieve optimization, knowledge and training gaps will have to be addressed in the adoption of a new paradigm and industry infrastructure based on digitized and interconnected enterprise systems that rely on computational power, communications technologies, cybersecurity, and advanced controls.
For example, to implement AI and other advanced technologies in pharmaceutical manufacturing, a variety of expertise beyond traditional biology, chemistry, and process engineering will be required. Data scientists, computational and systems engineers, IT specialists, and AI experts, for example, will most likely be needed. At least initially, regulators and business may be fighting for the same tiny pool of talent in these areas. New labor force training standards are undoubtedly on the way, and comprehensive training programs will be required.
There will also be a need to understand the operation of smart devices that will enable monitoring and operating from a distance by operators and supervisors. These technologies will simplify the changeover, setup, and maintenance and the life cycle management. There will also be a need for the workforce to operate cross functionally, integrating several disciplines.
Incorporating best practices and new technologies into the business is required. The difficult part is changing the culture and persuading individuals to accept a new way of doing business after having done things a certain way for many years. Organizational Change Management will be necessary (OCM). OCM is the “application of a systematic process and set of tools for driving the people side of change to accomplish a desired outcome,” according to Prosci, the global leader in change management best practices research. Additionally, strong communications, with a leadership team eager to drive positive experiences through technology, will be required.
Planning and Process Challenges
Because the potential for Pharma 4.0™ is so great and so wide-ranging, many pharmaceutical companies are racing to implement the technology and haven’t defined their primary goals and what problems they’re seeking to solve.
If these two important questions are not answered before starting down the path to Pharma 4.0™, the direction will be unclear, and many firms will lose sight of their objectives and goals. The organization’s business processes will undergo significant changes as a result of the major technologies required for digital transformation, and those changes must be acknowledged and understood before they are implemented. The company needs to have a strategy and a plan to get there.
Pharma 4.0™ and Validation 4.0 require an holistic control strategy. A strategy that follows the product from research to development, to tech transfer, and then through to commercial manufacturing. With this strategy there also needs to be a synergy between digital automation and guidelines and an enhancement of the quality manufacturing focus, where Quality Target Product Profiles are required for all products. With this control strategy, the data from machines and components is immediately available without traversing various systems . The operators’ remarks can be automatically recorded making the continuous improvement process easy to implement.
Technology Challenges
Transitioning to Pharma 4.0™ and leveraging Validation 4.0 guidelines requires incorporating new technologies and ensuring that everything works together to produce reliable data across the value chain. The technology needs to provide a means to integrate data and eliminate the silos that may exist throughout the organization today. These data silos can exist across process areas, applications being used, and organizational groups. A few examples are business quality data, IT quality data, product quality data, and supplier quality data. In many situations, the data is not being collected using the same method and this incongruent data has a negative impact on the decision process. Many times, this leads to inefficiencies and sometimes costly inaccuracies.
Organizations need to look at technology solutions across the value chain. From the company’s business partners and the customer perspective, the organization needs to be able to make informed decisions based on data compiled from various sources and to communicate specific aspects of that data accurately to suppliers, vendors, and customers.
Innovative technologies also necessitate new processes and more regulatory scrutiny. Manufacturers must remain nimble and able to adopt new skills in order to improve manufacturing and provide high-quality products.
To connect the essential instruments and equipment, the technology including tools, devices, and IT systems must be on an open platform with common data standards and, most likely, be cloud-based to enable data sharing. Integration and traceability, as well as automation, rely on information systems that eliminate needless manual or human interaction while also lowering regulatory scrutiny. Leveraging the right technology across the organization that is integrated and provides consistent and accurate information is imperative.
Operating within existing regulatory frameworks can be considered another challenge for Pharma 4.0™ and technical innovation when it comes to regulatory restrictions. Due to a lack of regulatory precedent, the industry may continue to use old practices even though new processes will lower overall regulatory burden and improve quality in the long run.
Summary
Moving to Pharma 4.0™ and incorporating Validation 4.0 guidelines impacts the entire organization. There are challenges that influence the success of an organization meeting its objectives with Pharma 4.0™. These challenges are in the areas of people, processes, and technology. To move to this new approach, it will require training of the people and a cultural shift to be successful. It will also involve an understanding of the objectives of the move to Pharma 4.0™ and the processes involved and an holistic control strategy. Additionally, with Pharma 4.0™, there is also the technology aspect. This requires an understanding of the new technologies so that everything works together to produce reliable data across the value chain.
Why it Matters to You
Before an organization embarks on an initiative to implement Pharma 4.0™ and Validation 4.0 guidelines into the business, there needs to be an understanding of the areas that could provide challenges. These challenges impact the success of the organization. In this blog, we discuss:
- The key challenges faced by organizations looking to implement this new approach.
- The impact of training and cultural on the move.
- What a Holistic Control Strategy role is in the implementation.
- Technology consideration when implementing this new approach.
About Astrix
For over 25 years, Astrix has been a market-leader in dedicated digital transformation & staffing services for science-based businesses. Through our proven laboratory informatics, digital quality & compliance, and scientific staffing services we deliver the highly specialized people, processes, and technology to fundamentally transform how science-based businesses operate. Astrix was founded by scientists to solve the unique challenges which science-based businesses face in the laboratory and beyond. We’re dedicated to helping our clients speed & improve scientific outcomes to help people everywhere.
Case Study: LabWare Centralized Data Review for a Global Biopharmaceutical Company
Overview A global biopharmaceutical company specializing in discovery, development,... LEARN MOREWhite Paper: Managing Data Integrity in FDA-Regulated labs.
New White Paper LEARN MORELET´S GET STARTED
Contact us today and let’s begin working on a solution for your most complex strategy, technology and staffing challenges.
CONTACT US


