
LIMS Data Migration: How to Ace the Journey from Planning to Post-Migration Evaluation
Data migration, at its core, appears deceptively simple—a process aimed at transferring data from an old system to a new one. When performed well, labs are better positioned to derive more value from their laboratory information management systems (LIMS) and their data. However, LIMS data migration can be quite complex and risky if not executed correctly. Between lost data, data format conversion complexities, data security concerns, and increased costs, LIMS data migration failures can threaten business continuity and push labs past the point of no return. Hence, understanding the complexities and following precise steps are crucial to ensure a successful migration that not only preserves data integrity but also enables enhanced operational efficiency and innovation.
What is LIMS data migration and why is it needed?
Data migration refers to the process of transferring data from one system or location to another. This could involve moving data from an old LIMS to a new one, upgrading to a newer version of the LIMS, or consolidating data from multiple sources into a single LIMS.
A myriad of factors driving business imperatives and technological evolution warrant the need for LIMS data migration. Primarily, the need for data migration arises from system upgrades or replacements, where laboratories seek to harness the capabilities of advanced LIMS platforms to improve efficiency and productivity. Similarly, vendor changes may prompt the migration, as laboratories move towards providers offering enhanced features or better support. Evolving compliance requirements may also necessitate data migration to avoid regulatory pitfalls. Moreover, as businesses expand, data centralization becomes imperative for streamlining operations, eliminating data silos, and facilitating collaboration across geographies. The adoption of cloud-based solutions not only enhances accessibility but also enables scalability and cost-effectiveness. Additionally, the demand for enhanced reporting and analytics drives laboratories towards platforms capable of delivering actionable insights from vast datasets. Legacy system decommissioning and security enhancements further propel the need for migration, as laboratories strive to mitigate risks and strengthen their data infrastructure against emerging threats.
Steps for a Successful Data Migration
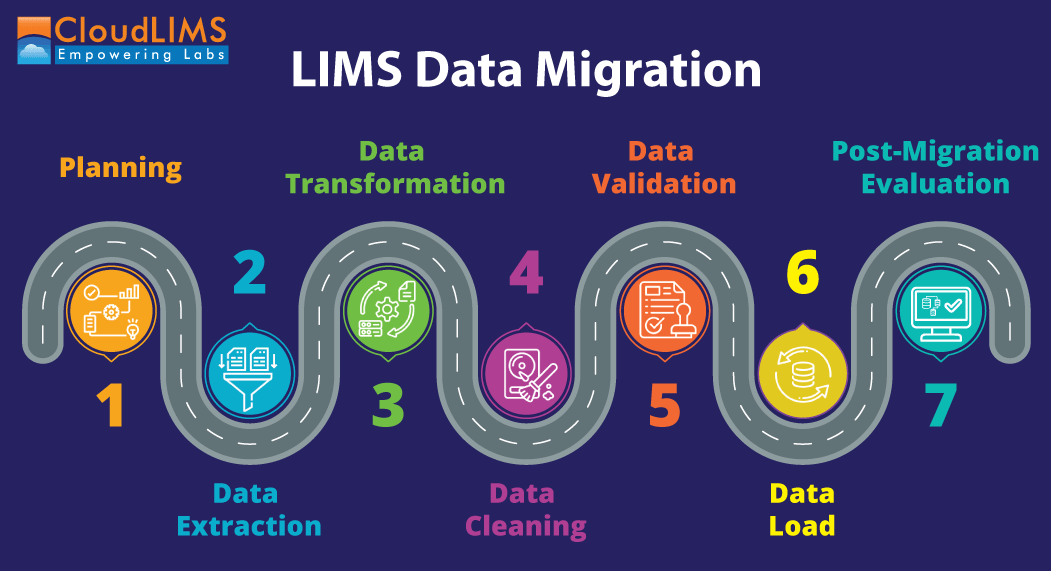
Figure 1: Steps for a successful LIMS data migration (Figure courtesy of CloudLIMS)
Executing a data migration exercise well involves following a set of steps and stages thoroughly.
Planning
The first crucial step in a LIMS data migration project is planning. It begins with the allocation of essential resources within the laboratory, ensuring that sufficient personnel, expertise, and technical infrastructure are available to support the migration process. Collaborating with a reliable LIMS vendor can significantly streamline this phase, as they can provide guidance and assign a dedicated project manager to oversee the entire migration journey. During the planning stage, several key considerations must be addressed to ensure a smooth and effective migration process.
- Stakeholder alignment is paramount, with all parties involved in the project having clearly defined goals aligned with the overarching objective of achieving a seamless transition. This involves identifying data owners and assembling specialized teams or task forces to manage specific aspects of the migration.
- Legacy data evaluation is another critical aspect of the planning stage that requires a thorough assessment of the data housed within the existing system. Laboratories must determine which data is essential for migration to the new system and whether certain information can be archived or stored in alternative repositories, such as data warehouses, to streamline the migration process.
- Data suitability is also a crucial consideration, ensuring that the data earmarked for migration is compatible with the new LIMS platform and meets the necessary quality standards.
- Regulatory compliance is another vital aspect to address during the planning stage, ensuring adherence to stringent security and compliance guidelines to maintain accreditations and certifications.
- Moreover, establishing a clear migration timeline that outlines the proposed schedule for the migration exercise and identifies strategies to minimize disruption to laboratory operations is essential. This involves careful coordination of activities to ensure a seamless transition while mitigating the risk of operational disruptions.
- An integral decision during the planning stage is the selection of the migration approach. Three common methods include the parallel approach, incremental approach, and the big bang approach.
- In the parallel approach, both the existing (legacy) LIMS and the new LIMS operate simultaneously for a defined testing period. This method is widely adopted by labs due to its ability to ensure consistency and accuracy in the new system. However, it’s crucial to note that this approach can be resource-intensive and complex. Deploying personnel to support two systems can incur significant expenses. Nevertheless, the benefits are notable, as this approach enhances the accuracy of the system. Additionally, having the old system available for reference during the transition period offers reassurance and allows data verification when needed.
- In the incremental method, data migration occurs gradually, often in phased stages rather than all at once. This approach involves a step-by-step migration process, minimizing the risk of disruptions and allowing laboratory operations to continue uninterrupted. For instance, in the initial phase, system configurations such as calculations can be addressed. Subsequently, instrument integration can be tackled in the second phase, followed by the integration of third-party software in the third phase. By breaking down the migration process into stages, laboratories can seamlessly implement the new system while maintaining normal operations.
- In the big bang approach, laboratory data and functionality are transferred from the existing system to the new system in a single, comprehensive operation. This transition occurs all at once, usually during a predefined cutover period. While this method offers speed, it is less commonly used due to its drawbacks such as significant downtime and disruption to customer service. Moreover, as data complexity and quantity increase, implementing the big bang approach becomes increasingly challenging.
Data Extraction
The data extraction step is an important step in the data migration process, involving the retrieval of data from the existing LIMS system in preparation for its transfer to a new LIMS or an updated version of the existing LIMS. This phase demands a comprehensive assessment to identify the specific data elements and records requiring migration, including diverse information ranging from sample details to test results and instrument data. Careful consideration is given to all data to be transferred, calling for a thorough audit of the source data. Labs need to identify the kind of data they have. For instance, transferring structured data, organized within tables and columns, is typically a smooth process, whereas transferring unstructured data, such as images, is more challenging. It’s important to have a LIMS that can support the export of large volumes of data in a simple and practical way. As the foundational step in the migration journey, proper data extraction lays the groundwork for a seamless transition to the new system.
Data Transformation
The data transformation or data mapping step in the data migration process is a crucial step where attributes from one database are matched to their counterparts in another using a predefined template. This process is essential for ensuring that data retains its integrity and structure during the transition to a new LIMS or a transformed version of an existing one. The complexity of data mapping can vary significantly, depending on factors such as the volume of data, the diversity of data types, and the disparities between the legacy data source and the new LIMS. Complex mappings may involve transformations to reconcile differences in data formats, field names, or data structures, while simpler mappings may entail straightforward one-to-one mappings. Regardless of complexity, extensive attention to detail is required to ensure accurate data transfer and minimize the risk of data loss or corruption.
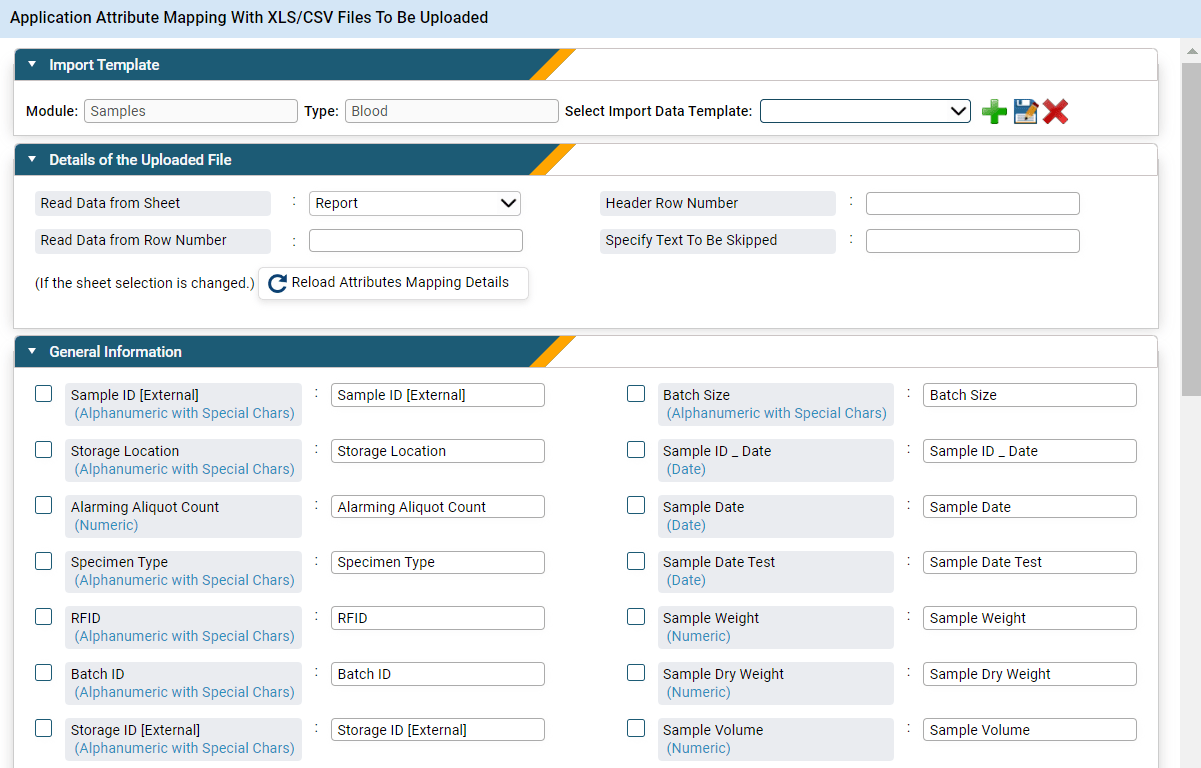
Figure 2: Leverage a good LIMS for seamless data transformation using its attribute mapping capabilities.
Data Cleaning
The data cleaning step of the data migration process is a critical one aimed at enhancing the quality and reliability of datasets slated for transfer to a new LIMS. This phase involves an examination of datasets, tables, and databases to identify and rectify various anomalies, including unreliable, inaccurate, duplicated, or outdated information. Through rigorous error detection and rectification, data cleaning mitigates the risk of transferring errors and inaccuracies to the new system, safeguarding data integrity. Key tasks within the data cleaning process include identifying and rectifying errors such as spelling discrepancies, inaccuracies, or incomplete information within the dataset. Additionally, removing duplicate data and standardizing data formats, units, and structures to align with the specifications of the new LIMS are essential. Furthermore, data integrity checks and normalization procedures are performed to validate data consistency and adherence to predefined standards.
Data Validation
The data validation step is aimed at verifying the accuracy, consistency, and compliance of data transferred from the old system to the new system. This rigorous process ensures that the migrated data is devoid of potential errors and discrepancies. Through systematic validation procedures, the data is examined to identify any anomalies or inconsistencies that may have arisen during the migration process. Key aspects of data validation include verifying the completeness and correctness of transferred data, ensuring that all essential information has been accurately migrated. Additionally, consistency checks are conducted to ascertain that data formats, units, and structures conform to the specified requirements of the new LIMS.
Data Load
The data load step in the data migration process marks the culmination of the journey, where databases, tables, or structures of the new LIMS are populated with the extracted, transformed, and validated data from previous stages of migration. This phase represents the final bridge between the old and new systems. Through the execution of data loading procedures, the integrity and accuracy of the migrated data are preserved, ensuring that the new LIMS is equipped with a robust foundation of reliable information. Key considerations during this phase include optimizing data loading processes to minimize downtime and disruption to laboratory operations, as well as implementing mechanisms to monitor and verify data integrity post-loading.
Post-Migration Evaluation
Post-migration evaluation ensures that the migrated data aligns with expectations, meets stringent quality standards, and facilitates the effective operation of the new LIMS. This critical phase includes a comprehensive validation process to affirm migration success, preserve laboratory data integrity, and promptly address any emerging issues encountered or observed during the transition. Central to this evaluation is a data integrity check to verify the completeness and accuracy of all data transferred from the old system to the new system. Subsequently, the functionality of the new LIMS is examined to confirm its seamless operation, This is done with assessments such as user acceptance testing (UAT), performance monitoring, and validation of customizations. Custom configurations or modifications are particularly tested to ensure they enhance workflow efficiency without compromising overall system performance. A thorough post-migration evaluation instills confidence in the reliability and effectiveness of a lab’s new LIMS, resulting in improved operational efficiency and productivity.
Conclusion
As technology continues to evolve, laboratories increasingly seek more advanced LIMS solutions to streamline operations and drive innovation. Adopting these systems often requires a data migration exercise to transition from legacy platforms to modern, feature-rich LIMS. As outlined in the various steps of the LIMS data migration process, from planning to post-migration evaluation, it’s evident that data migration is a complex task that demands careful consideration and precise execution. Each stage, whether it’s data extraction, transformation, cleaning, or validation, plays a crucial role in ensuring a seamless data transfer while preserving integrity and accuracy. Moreover, the post-migration evaluation step serves as a final checkpoint, affirming migration success and validating the operational efficacy of the new LIMS. Ultimately, each step in the data migration process serves as a vital building block, fitting seamlessly together like pieces in a tangram, empowering laboratories on their digitization journey towards greater efficiency, innovation, and scientific discovery.
About CloudLIMS:
CloudLIMS.com is an ISO 9001:2015 and SOC 2-certified informatics company. Their SaaS, in-the-cloud Laboratory Information Management System (LIMS), CloudLIMS, offers strong data security, complimentary technical support, instrument integration, hosting and data backups to help biorepositories, analytical, diagnostic testing and research laboratories, manage data, automate workflows, and follow regulatory compliance such as ISO/IEC 17025:2017, GLP, 21 CFR Part 11, HIPAA, ISO 20387:2018, CLIA, ISO 15189:2012, and ISBER Best Practices at zero upfront cost. Their mission is to digitally transform and empower laboratories across the globe to improve the quality of living.
About the Author
Arun Apte, CEO, CloudLIMS
Arun Apte is a serial entrepreneur and laboratory research scientist specializing in bioinformatics. He founded CloudLIMS in 2014, bringing the benefits of a SaaS LIMS to the laboratory market. This has enabled hundreds of small labs including biorepositories, clinical research and diagnostic labs, and analytical testing labs, including food & beverage, cannabis, environmental, water, and material testing labs to gain the efficiency of a LIMS previously only affordable to large laboratories. Arun is an invited speaker at conferences all over the world. Recently, he was an invited speaker at the Cannabis Quality Conference 2023.
Prior to founding CloudLIMS, he founded PREMIER Biosoft forging strategic partnerships with Thermo, Agilent, SCIEX, and other mass spectrometry instrument companies. Apte holds a B.A. in molecular and cell biology and biophysics from the University of California at Berkeley. He has published extensively on bioinformatics.
About Astrix
Astrix partners with many of the industry leaders in the informatics space to offer state of the art solutions for all of your laboratory informatics needs. Through world-class people, process, and technology, Astrix works with clients to fundamentally improve business, scientific, and medical outcomes and the quality of life everywhere. Founded by scientists to solve the unique challenges of the life science community, Astrix offers a growing array of fully integrated services including LIMS Consulting Services designed to deliver value to clients across their organizations.
Case Study: LabWare Centralized Data Review for a Global Biopharmaceutical Company
Overview A global biopharmaceutical company specializing in discovery, development,... LEARN MOREWhite Paper: Managing Data Integrity in FDA-Regulated labs.
New White Paper LEARN MORELET´S GET STARTED
Contact us today and let’s begin working on a solution for your most complex strategy, technology and strategic talent services.
CONTACT US



