
Agile Regulatory Compliance: How quickly do your current processes allow you to respond to regulatory changes?
Staying current with ever-changing regulatory compliance regulations can be an overwhelming and time consuming task for life science organizations. With changes released nearly every day, if you’re not referencing the most recent guidance documents, you may be introducing unnecessary compliance risks, including citations, fines, and failed submissions – ultimately wasting valuable time, money and resources.
Throughout the drug development lifecycle, it is critical to have a comprehensive and accurate view of today’s regulatory landscape. Additionally, the emergence of new technologies has expanded the scope and frequency of change to regulatory requirements. Monitoring ongoing daily reports and a vast array of government website may offer moderate success in obtaining all of the necessary information regarding updated compliance guidance. However, the challenge still lies in translating those changes to the everyday workflow and processes that occur within the laboratory and manufacturing environment.
The Agile Response to Regulatory Change
The key question to address is, ‘how quickly do your current processes allow you to respond to regulatory changes’? Effective regulatory change management requires an agile process to administer the consistent influx of new guidelines in a cohesive and timely manner. Global and multi-site operations have an even larger complexity of harmonizing regulatory change and integrated policy management across all regions of the organization.
The response to this challenge is agile, real-time compliance. To achieve regulatory efficiency throughout all areas of your organization, real-time compliance management can provide a sustainable solution to this complex process. Clarivate’s Regulatory Intelligence Tracking App (ClaRITA), is a business-specific regulatory monitoring and impact assessment platform that provides pharmacovigilance, safety, clinical, and technical operations teams the information they need to monitor changing legislation and regulations and remain in compliance.
ClaRITA is a web-based regulatory workflow solution that interacts with the Cortellis Regulatory Intelligence™ data to effectively manage the regulatory actioning lifecycle. Through the continuous daily surveillance of regulatory news worldwide, ClaRITA enables you to streamline and automate regulatory monitoring and impact assessments to facilitate real-time compliance in the following ways:
- Provides a single source of truth for regulatory updates
- Issues automated daily alerts regarding new and important regulatory changes and priority classifications
- Processes daily Cortellis Regulatory Intelligence (CRI) updates to obtain comprehensive, global, and expertly-analyzed information that spans all regulatory functions across the R&D lifecycle
- Requires user authentication and allows for role-based access control for GxP compliance
- Informs users of pre-publication Triage and Impact Assessment to determine the potential impacts of regulatory proposals
- Manages assigned tasks, user access to tasks, and actioning of tasks
- Reports performance on task resolution and trending by groups and users
- Provides collaboration and status tracking tools
Streamlining the regulatory alert process allows you to efficiently manage and track responses to regulatory changes, increasing transparency across the organization to ensure that tasks are acted upon by required teams within the needed timeframe.
Example ClaRITA workflow and document status assignment
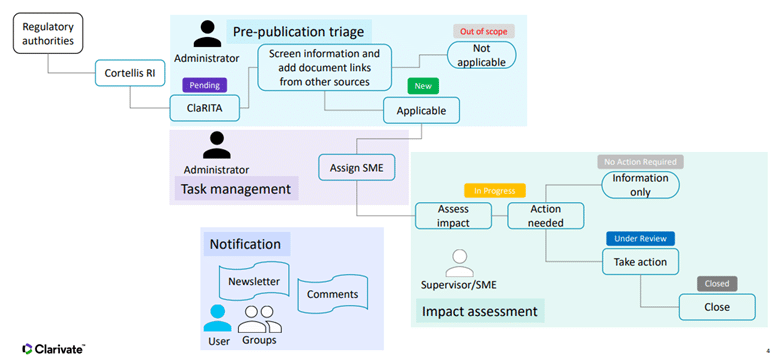
Conclusion
It is of critical importance for regulated industries to comply with the most current regulatory guidelines. Having a real-time compliance strategy in place that allows for automated regulatory compliance monitoring and an agile change management approach to quickly implement and track performance on these tasks will significantly streamline the regulatory actioning lifecycle across your organization.
Why it Matters for You
Throughout the drug development lifecycle, it is critical to have a comprehensive and accurate view of today’s regulatory landscape. Effectively managing the ever-change compliance regulations is a daunting, time consuming task for life science organizations. An agile, real-time compliance management strategy can provide a sustainable solution to this complex process in the following ways:
- Adopting an agile regulatory change management strategy will enable you to achieve regulatory efficiency throughout all areas of your organization.
- Streamlining the regulatory alert process will allow you to efficiently manage and track responses to regulatory changes, increasing transparency across the organization to ensure that tasks are acted upon by required teams within the needed timeframe.
- Implementing Clarivate’s Regulatory Intelligence Tracking App (ClaRITA) will provide a business-specific regulatory monitoring and impact assessment platform that equips pharmacovigilance, safety, clinical, and technical operations teams with the information they need to monitor changing legislation and regulations and remain in compliance.
- Continuously monitor regulatory news across the globe on a daily basis using Cortellis Regulatory Intelligence (CRI) provides automated updates to obtain comprehensive, and expertly-analyzed information that spans all regulatory functions across the R&D lifecycle.
About Astrix
Astrix partners with many of the industry leaders in the informatics space to offer state of the art solutions for all of your laboratory informatics needs. With over 25 years of industry proven experience, Astrix has the informatics specialists and business process analysis tools required to develop and implement the solution that works best for your enterprise. Our domain experts have helped hundreds of companies globally effectively navigate their digital transformation journey, connecting people, processes and systems to accelerate the advancement of science and medicine.
Case Study: LabWare Centralized Data Review for a Global Biopharmaceutical Company
Overview A global biopharmaceutical company specializing in discovery, development,... LEARN MOREWhite Paper: Managing Data Integrity in FDA-Regulated labs.
New White Paper LEARN MORELET´S GET STARTED
Contact us today and let’s begin working on a solution for your most complex strategy, technology and staffing challenges.
CONTACT US



