
Structured Content Authoring (SCA) in Clinical and Regulatory Documents
In our previous post, we explored how AI is transforming regulatory and clinical documents, moving from hype to real, measurable value. In this second installment, we take a closer look at Structured Content Authoring (SCA), a foundational capability that enables reuse, traceability, and automation at scale.
SCA is more than a technical upgrade. It is a fundamental precursor to AI enabled content generation. In an industry where time to market is measured in millions of dollars per day, streamlining this workflow through automation to deliver submission-ready documents faster is a top priority for pharmaceutical, biotech, and medical device companies.
What are the core components of SCA?
The essence of SCA in clinical and regulatory documents is the accurate and consistent presentation of clinical data, we have compiled these components in the following illustration:
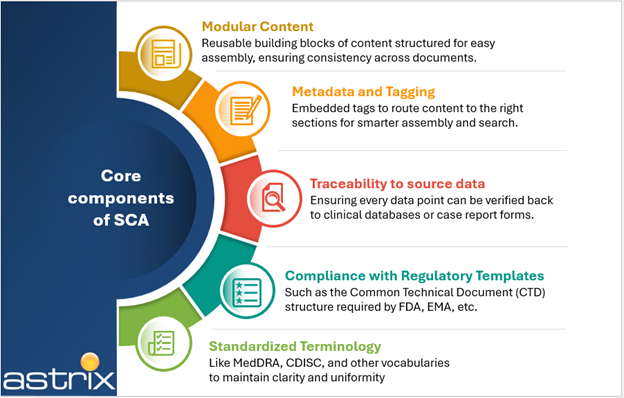
This often involves multiple stakeholders and source data systems that need to conform to the demands of the regulatory requirements, (e.g., EMA, FDA, ICH guidelines) across a complicated volume of different document types: protocols, CSRs, IBs, INDs, NDAs. Quality data is critical for producing structured content outputs, namely Tables, Figures and Listings—as well as effective narratives and summaries, which are critical for Adverse Event and Protocol Deviation reporting.
How is SCA evolving with technology?
The current wave of SCA solutions enable structured authoring through metadata tagging and smart templates. These tools help automate document assembly and ensure traceability to the data sources.
This includes:
- Smart Templates define the reusable content components and adapt documents for specific regulatory requirements.
- Metadata tagging helps guide data to the correct section of the document.
- Traceability features ensure content are sourced from validated datasets, supporting compliance and quality.
The content authoring technology reduces manual input and helps ensure consistency and completeness across all documents.
What do you need to know before implementing a solution?
Choosing the right SCA solution is only part of the equation. These 3 factors are critical to successful implementation.
1. Stakeholder alignment and change management
Before evaluating tools, align teams across regulatory, medical writing, data management, clinical operations, and IT. This early alignment helps define shared goals and establishes the key requirements and functionality for a successful vendor evaluation. It also sets the stage for smoother adoption and long-term sustainability.
2. Enterprise Data Lake: manageable data repository
To enable an automated population of structured content, organizations must establish a robust data foundation. This includes:
- Data provenance to track the origin and transformation of information
- Metadata tagging to link datasets to the correct parts of a document
An enterprise data lake with these capabilities acts as the backbone of any effective SCA program.
3. Key considerations: tools, governance, training
Even the best technology needs a dedicated support system. Success depends on:
- Governance frameworks to define ownership and processes
- Training to build user confidence and consistency
- Regular evaluations to identify redundant tools and streamline workflows
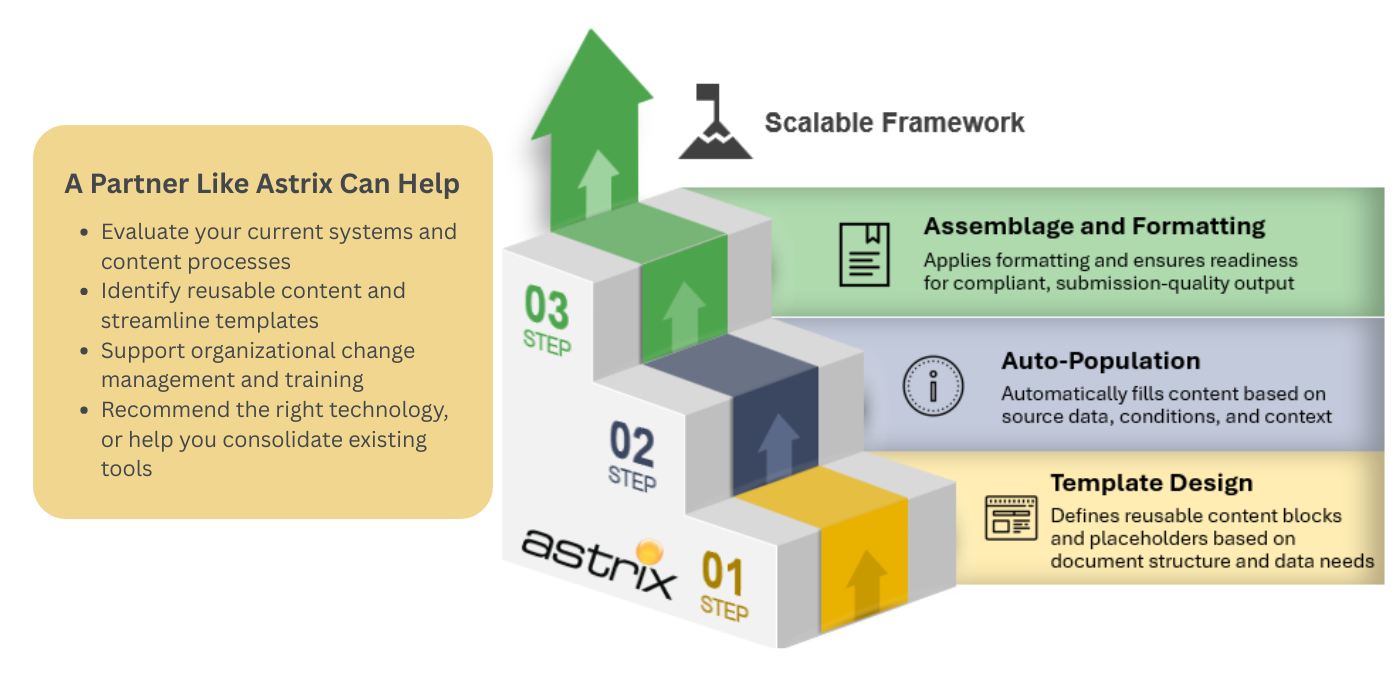
Pro Tip: Work with experienced partners who can guide your team through implementation. Astrix offers a tailored methodology that helps clients build scalable, structured content workflows.
What are the regulatory bodies foreseeing as the future of digitized and automated structured content?
Structured Content Authoring aligns with the current regulatory guidance on data integrity, traceability, and efficient reuse of content to improve submission quality and review readiness.
While agencies like the FDA, EMA, MHRA, and NIH have not explicitly mandated SCA, their growing emphasis on structured data, interoperability and digital transformation strongly supports the adoption of SCA principles in clinical and regulatory documentation. An NIH article on structured content and data management highlighted the potential of Health Level 7 Fast Healthcare Interoperability Resources (HL7 FHIR) for standardization:
“In addition to FDA’s and EMA’s efforts to develop FHIR-compliant data standards, a new HL7 FHIR project was established in late 2022 with the goal of developing global internal data standards to enable exchange of data across biopharmaceutical industry data systems.”
Structured content is central to this evolution. It enables better data quality, supports interoperability, and allows faster review by health authorities.
What does this mean for your Structured Content Authoring future?
SCA is not a plug-and-play solution. It requires strategy, alignment, and long-term planning. A partner like Astrix can help get you started.
We welcome any opportunity to engage with your key stakeholders and subject matter experts on structured content authoring. Advancing this effort effectively requires strategic alignment across your cross-functional teams and a sound understanding of the solution landscape. From strategy to adoption, our dedicated experts are here to troubleshoot issues, answer questions, and guide you through each step.
Contact us to learn how we can help you master SCA within your organization.
About Astrix
Astrix is the unrivaled market-leader in creating & delivering innovative strategies, solutions, and people to the life science community. Through world class people, process, and technology, Astrix works with clients to fundamentally improve business & scientific outcomes and the quality of life everywhere. Founded by scientists to solve the unique challenges of the life science community, Astrix offers a growing array of strategic, technical, and staffing services designed to deliver value to clients across their organizations.
Coming Soon: Part Three of our blog series examines what AI-enabled content generation truly entails and where we are today with this technology. We will include specific guidance on how to choose an AI solution for Clinical and Regulatory content generation and successfully implement the solution.
Citation: Beierle J, Algorri M, Cortés M, Cauchon NS, Lennard A, Kirwan JP, Oghamian S, Abernathy MJ. Structured content and data management-enhancing acceleration in drug development through efficiency in data exchange. AAPS Open. 2023;9(1):11. doi: 10.1186/s41120-023-00077-6. Epub 2023 May 8. PMID: 37193559; PMCID: PMC10164450.
Case Study: LabWare Centralized Data Review for a Global Biopharmaceutical Company
Overview A global biopharmaceutical company specializing in discovery, development,... LEARN MOREWhite Paper: Managing Data Integrity in FDA-Regulated labs.
New White Paper LEARN MORELET´S GET STARTED
Contact us today and let’s begin working on a solution for your most complex strategy, technology and strategic talent services.
CONTACT US



