
Decode Compliance and Quality Standards Requirements with a Water LIMS
Water quality directly impacts public health and the environment, which means maintaining stringent standards in water testing is non-negotiable. The stakes are significant, and even a single error can result in substantial fines and may jeopardize public health and safety. For example, failing to comply with regulations like the Clean Water Act (CWA) can lead to hefty fines for each violation. This underscores the critical importance of adhering to complex regulations, such as EPA guidelines, and standards, such as ISO/IEC 17025. Navigating these complex requirements is no easy task, especially when labs are bogged down by challenges such as managing samples and records, maintaining end-to-end traceability, and prepping for audits. To meet compliance and standards, laboratories need more than just traditional methods; they need the right tools. Informatics tools offer a strategic advantage by automating processes, securing data integrity, and simplifying compliance. In this article, we will explore how informatics tools, such as a Water LIMS, a Laboratory Information Management System (LIMS) for water testing, can be the key to achieving regulatory compliance, enhancing operational efficiency, and upholding the highest standards in water testing.
What Are the Essential Compliance and Standards for Water Testing Laboratories?
Here is a detailed overview of the compliance and standards that water testing laboratories must follow.
1. ISO Standards: Various ISO standards are relevant to water testing. ISO 17381:2003 provides guidance on choosing ready-to-use analysis methods, focusing on quick and cost-effective techniques. ISO 5667-1:2023 outlines procedures for sampling water, including wastewater, sludges, effluents, suspended solids, and sediments. This is to ensure that samples are collected in a way that accurately reflects water quality. Meanwhile, ISO 17025 offers detailed guidance on upholding rigorous quality standards in testing and calibration laboratories.
While ISO compliance is not mandatory, adhering to these standards can significantly enhance the reliability and credibility of laboratory results, benefiting both the laboratory and its stakeholders.
2. Safe Drinking Water Act (SDWA): The SDWA sets standards for the quality of drinking water in the U.S. It sets contaminant limits and mandates continuous monitoring to ensure water safety and safeguard public health. It also mandates that the EPA oversees a certification program for laboratories conducting drinking water tests, ensuring that these labs use only EPA-approved testing methods. This framework ensures the reliability and accuracy of water quality assessments, thereby protecting public health.
3. 21 CFR Part 11: This FDA regulation sets standards for electronic records and signatures, ensuring that digital documentation in laboratories is secure and accurate, and effectively replaces paper-based documentation and handwritten signatures.
4. Information Collection Requirements (ICR) for Drinking Water Laboratories: The ICR targets large-scale water sources, specifically surface water bodies serving over 100,000 people and groundwater bodies serving more than 50,000 people. Established by the EPA, this rule aims to gather and analyze data on the risks and benefits associated with disinfecting drinking water. To ensure compliance, ICR also outlines specific laboratory approval requirements for conducting these essential tests. These requirements dictate the procedures for collecting and reporting data related to drinking water quality, ensuring consistency and accuracy in the information provided by laboratories.
5. UCMR Laboratory Approval Program: The UCMR 5 Laboratory Approval Program applies to all laboratories that analyze samples under the fifth Unregulated Contaminant Monitoring Rule (UCMR 5). The EPA established the UCMR 5 to oversee the collection and testing of water samples for 30 chemical contaminants, utilizing methods developed by the EPA and other recognized organizations. To ensure compliance, the EPA has provided a detailed manual outlining the approval requirements and standards for laboratories participating in UCMR 5.
6. NELAP: The National Environmental Laboratory Accreditation Program (NELAP), developed by The NELAC Institute (TNI), establishes uniform standards for environmental testing laboratories. These voluntary, consensus-based standards aim to enhance laboratory services by ensuring the generation of high-quality data in water testing. NELAP offers a structured framework for accrediting laboratories that analyze a wide range of environmental media, including water, soil, air, and other substances.
7. CALA: CALA, which stands for the Canadian Association for Laboratory Accreditation, is a recognized organization that provides accreditation services to water testing laboratories across Canada. It ensures that laboratories meet high standards of quality and competence by evaluating their compliance with relevant industry standards, such as ISO 17025. CALA’s rigorous assessment process includes proficiency testing, internal audits, and continual improvement protocols, all of which contribute to high levels of precision and reliability in water quality analysis. CALA’s accreditation programs cover a broad range of testing, calibration, and inspection services, including those related to environmental testing, cannabis testing, and more. CALA conducts reassessments of accredited laboratories every two years to confirm their ongoing adherence to requirements.
8. UK Environment Agency: In the UK, the Environment Agency (EA) oversees various activities and operators to safeguard the environment and public health. The EA requires drinking water to be tested rigorously for various contaminants, including microorganisms, chemicals like nitrates and pesticides, and metals such as lead and copper. The appearance and taste of the water are also closely monitored. To ensure public safety at bathing sites, the EA employs environmental officers who collect water samples and primarily test for two types of bacteria: E. coli and intestinal Enterococci. The results are made publicly available. Additionally, the EA monitors discharges into water bodies to protect environmental quality.
To ensure that laboratories provide accurate and reliable results, the EA has established strict requirements for water testing facilities. These requirements include accreditation to ISO/IEC 17025, the use of proper sampling and sample handling methodologies, validation of test methods, participation in proficiency testing, and accurate data reporting.
9. ASTM Standards: The American Society for Testing and Materials (ASTM) provides guidelines for evaluating the methods and facilities used to analyze water quality and detect contaminants. These standards ensure that water testing, conducted by local government authorities, water distribution facilities, and environmental laboratories, meets the necessary health, security, and environmental requirements, ultimately ensuring safe water consumption. The standards span a plethora of methods such as water sampling methods, standard test methods for inorganic constituents such as silver, bromate, bromide, chlorate, and chloride in water, methods for analysis for organic substances and radiochemicals in water, and many more.
10. 40 CFR Part 141: 40 CFR Part 141 sets forth the primary regulations for drinking water quality under the Public Health Service Act, as revised by the Safe Drinking Water Act. These regulations apply to public water systems in the U.S. and are designed to safeguard public health by ensuring safe drinking water. According to the standard, larger community water systems (those serving 75,000 or more people), must start monitoring within a year after new standards are implemented, with full compliance required within two years. Smaller systems, serving between 10,000 and 75,000 people, have a slightly longer timeline, with monitoring beginning within three years and full compliance required within four years.
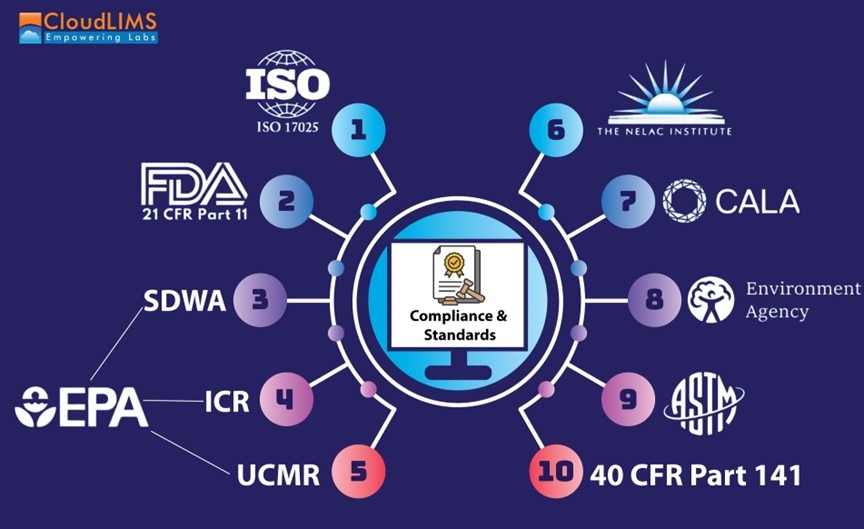
Figure 1: A schematic representation of regulatory compliance and standards for water testing labs (Figure courtesy of CloudLIMS)
What are the Challenges Faced by Water Testing Labs in Maintaining Compliance and Standards?
The risks associated with non-compliance are significant. Beyond financial penalties, non-compliance can cause substantial damage to reputation and public health, eroding trust with clients and the public. Water testing labs must ensure they remain compliant, protect their reputation, and steer clear of lawsuits and sanctions that can threaten their very existence. However, maintaining compliance in water testing laboratories is no small feat, especially given the complex regulatory environment and the high stakes involved. These labs face several challenges that can make compliance a complex task.
- Data Management: One of the most significant hurdles is managing the vast amount of data generated daily. Laboratories must handle everything from test results and calibration records to customer information and quality control data. It is essential for compliance that this data is accurate, securely stored, and readily accessible. However, relying on manual data entry can result in mistakes, inconsistencies, and missing information, which can undermine compliance efforts.
- Traceability: Traceability is another crucial aspect of compliance. Labs must be able to track every sample from collection to analysis and final reporting. This requires immaculate record-keeping and the ability to trace back results to their source at any point in time. Without a robust system in place, maintaining this level of traceability can be overwhelming, leading to potential lapses in compliance.
- Audit Readiness: Regular audits by regulatory bodies are a standard part of operations for water testing labs. Being audit-ready means having all necessary documentation, data, and processes in place and easily accessible at all times. Preparing for audits can be time-consuming and stressful, particularly if records are scattered across different systems or maintained in paper form. Any discrepancies or missing information during an audit can result in severe penalties, and labs can lose their accreditation status if no remedial measures are promptly taken.
How Does a Water LIMS Support Compliance and Standards in Testing Labs?
Water testing laboratories face constant pressure to stay compliant with numerous regulatory requirements. The powerful features of a water LIMS can help them meet compliance and quality standards with ease.
- Automated Data Management: One of the most powerful features of a water LIMS is its ability to automate data management. A LIMS captures and records data directly from instruments and other sources, minimizing human error and ensuring data is accurate, consistent, and securely stored.
- Sample Tracking and Traceability: A LIMS maintains a comprehensive chain of custody for samples, a key requirement in water testing laboratories. The system tracks the custodian of each sample from the moment it is collected, through analysis, to final reporting. This traceability ensures sample integrity and helps meet stringent regulatory demands for transparency and accountability.
- Audit Trails and Reporting: Compliance with regulations often requires detailed documentation of every step taken in the laboratory. A water LIMS automatically generates comprehensive audit trails, capturing who did what, when, and how. These trails are invaluable during audits, providing clear evidence of compliance. Additionally, a LIMS allows for the creation of customizable reports to meet specific regulatory requirements, making it easier to provide the necessary documentation during inspections or audits.
In addition to data management and traceability, a water LIMS also enhances compliance through robust document control and quality control (QC) measures. Document control ensures that all regulatory and operational documents, such as standard operating procedures (SOPs), protocols, and manuals, are centrally managed, up-to-date, and easily accessible. This prevents the use of outdated procedures and supports consistent laboratory practices. SOP management allows labs to enforce standardized processes across all testing activities, reducing the risk of errors and ensuring uniformity in operations. Additionally, integrated QC measures in a LIMS help monitor and evaluate the quality of results, automatically flagging any deviations and ensuring that testing meets the highest standards of accuracy and reliability.
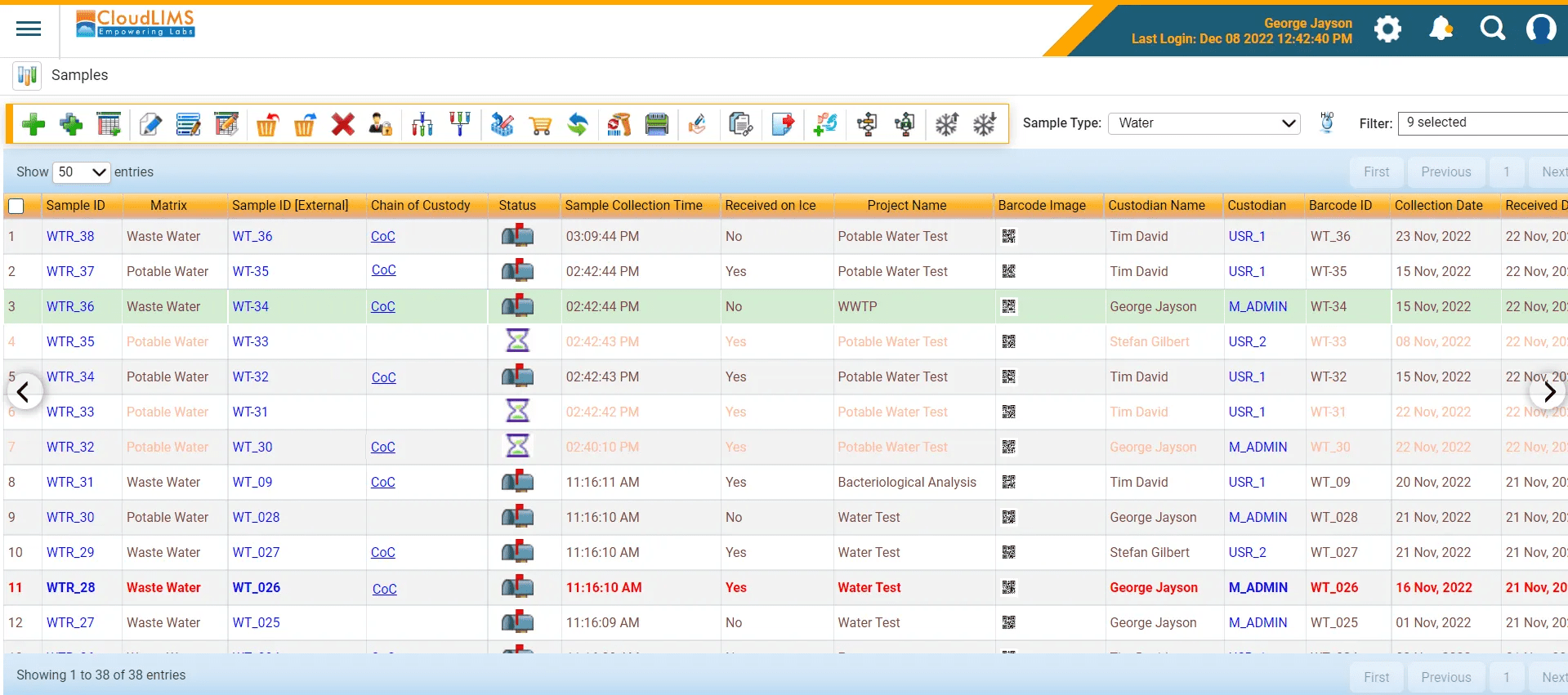
Figure 2: Manage samples and maintain a CoC with ease using a water LIMS (Figure courtesy of CloudLIMS)
Conclusion
Water testing laboratories are integral to safeguarding public health and the environment. For these labs, the complexities of managing vast amounts of data, maintaining traceability, and preparing for audits can be overwhelming, but these are challenges that must be met to uphold the integrity of testing processes and test results. Implementing a water LIMS transforms these challenges into manageable tasks, streamlining operations and providing the tools necessary to meet and exceed regulatory and quality requirements. With automated data management, sample traceability, audit trails, document management, and QC checks, a LIMS not only supports compliance but also enhances the overall efficiency and reliability of water testing laboratories.
About CloudLIMS
CloudLIMS.com is an ISO 9001:2015 and SOC 2-certified informatics company. Their SaaS, in-the-cloud Laboratory Information Management System (LIMS), CloudLIMS, offers strong data security, complimentary technical support, instrument integration, hosting and data backups to help biorepositories, analytical, diagnostic testing and research laboratories, manage data, automate workflows, and follow regulatory compliance such as ISO/IEC 17025:2017, GLP, NELAC, and 21 CFR Part 11, at zero upfront cost. Their mission is to digitally transform and empower laboratories across the globe to improve the quality of living.
About the Author
Arun Apte, CEO, CloudLIMS
Arun Apte is a serial entrepreneur and laboratory research scientist specializing in bioinformatics. He founded CloudLIMS in 2014, bringing the benefits of a SaaS LIMS to the laboratory market. This has enabled hundreds of small labs including biorepositories, clinical research and diagnostic labs, and analytical testing labs, including food & beverage, cannabis, environmental, water, and material testing labs to gain the efficiency of a LIMS previously only affordable to large laboratories. Arun is an invited speaker at conferences all over the world.
Prior to founding CloudLIMS, he founded PREMIER Biosoft forging strategic partnerships with Thermo, Agilent, SCIEX, and other mass spectrometry instrument companies. Apte holds a B.A. in molecular and cell biology and biophysics from the University of California at Berkeley. He has published extensively on bioinformatics.
About Astrix
Astrix partners with many of the industry leaders in the informatics space to offer state of the art solutions for all of your laboratory informatics needs. Through world-class people, process, and technology, Astrix works with clients to fundamentally improve business, scientific, and medical outcomes and the quality of life everywhere. Founded by scientists to solve the unique challenges of the life science community, Astrix offers a growing array of fully integrated services designed to deliver value to clients across their organizations.
Case Study: LabWare Centralized Data Review for a Global Biopharmaceutical Company
Overview A global biopharmaceutical company specializing in discovery, development,... LEARN MOREWhite Paper: Managing Data Integrity in FDA-Regulated labs.
New White Paper LEARN MORELET´S GET STARTED
Contact us today and let’s begin working on a solution for your most complex strategy, technology and strategic talent services.
CONTACT US



